New Drug Screening Tool for Cancer Research Comes to Market
ROCHESTER, N.Y., September 6, 2019 (Newswire.com) - Sanatela AT Medical Solutions, Inc. (“Sanatela” or the “Company”), an ATVentureCenter firm co-founded by Michael Crowley and Erin Crowley Ellis, is pleased to announce the development and production of the SanatelaTM Matrix -- a revolutionary new tool for precision cancer drug screening which will also provide new techniques for high-throughput drug screening.
The Matrix is an advanced biomaterial tissue that promises to profoundly improve the screening and testing of new and existing treatments against deadly Cancer Stem Cells (CSCs) in vitro while providing High-Throughput Drug Screening companies and research facilities with a new tool to directly target cancer and other stem cells.
Sanatela’s bioengineering process produces a 3D structure with all the components needed for expanding and maintaining stem cells, making it the ideal natural environment for screening chemotherapy drugs. The Matrix also has promising translational research applications including promoting burn, wound, bone and hair regeneration and repair.
Before the Company makes matrix sheets and test kits generally commercially available later this year, Sanatela intends to make its matrix sheets and test kits available on a pre-order only basis to select research hospitals, cancer and stem cell centers and High-Throughput Drug Testing firms seeking to perform research studies on this new and highly innovative technology. For more information, contact info@sanatelamedical.com.
What is the Sanatela Matrix?
The Sanatela Matrix employs technology developed at the University of Rochester Medical Center and exclusively licensed to the Company on a worldwide basis. It was co-invented by two faculty members in the University of Rochester’s School of Medicine and Dentistry--Omar S. Aljitawi, M.B.B.S., a researcher and practicing oncologist at Wilmot Cancer Institute who serves as Associate Professor of Medicine, and Hani Awad, Ph.D., a researcher focused on musculoskeletal tissue engineering and a Professor of Biomedical Engineering, of Orthopedics, and in the Center for Musculoskeletal Research.
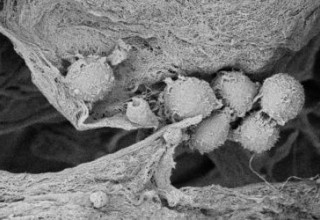
The Sanatela Matrix is a bioengineered tissue platform made entirely of Wharton’s Jelly -- an umbilical cord tissue obtained from live births that is normally discarded as medical waste. The Matrix is processed using a proprietary protocol to produce a porous, biodegradable and biocompatible scaffold that retains most of the extracellular matrix proteins, glycosaminoglycans and growth factors found in native Wharton’s Jelly. It can be produced in different forms and sizes, and can be produced in multi-well plate formats, making it capable of supporting high-throughput pharmaceutical screens and scientific studies.
The Wharton’s Jelly tissue is processed by Sanatela using a proprietary protocol to produce a porous, biodegradable and biocompatible matrix that retains most of the extracellular matrix proteins, glycosaminoglycans and growth factors found in native Wharton’s Jelly. The Sanatela Matrix includes an abundance of extracellular matrix components, such as collagen, fibronectin, hyaluronic acid, and sulfated proteoglycan, as well as a number of growth factors, including insulin-like growth factor 1 (IGF -1), fibroblast factor (FGF), transforming growth factor beta 1 (TGF-β1), epidermal growth factor (EGF), and platelet-derived growth factor (PDGF).
Using the Matrix to combat Cancer Stem Cells - Video https://youtu.be/XD34QkJTIcI
The stem cell theory of cancer proposes that, among all cancerous cells, a rare population act as stem cells that reproduce themselves and sustain the cancer, much like normal stem cells normally renew and sustain our organs and tissues.
The discovery of Cancer Stem Cells in some blood and tumor cancer types has ushered in a new era of cancer research and potentially new methodologies of individualized cancer treatment that could potentially cure cancer without the toxicities that are common in most current treatments. Some researchers even refer to cancer as a stem cell disease. The development of the Sanatela Matrix positions the company at the forefront of translational CSC research.
Many cancer researchers now believe this small population of CSCs are responsible for the reproduction and growth of cells within a cancer and that finding ways to kill them may be the key to effectively prevent cancer metastasis and relapse. If oncologists can eliminate cancer stem cells in the tumor, they will be able to treat cancer in a more effective and targeted manner.
“To successfully treat leukemia, you have to get rid of leukemia stem cells. If you don’t, the disease will just come back again,” says Dr. Omar S. Aljitawi, the Sanatela Matrix co-inventor. “The Matrix enables us to closely examine what a cancer stem cell is so we can treat it effectively.”
Screening cancer treatments with the Matrix
When Sanatela researchers place a 6mm “disk-like” segment of the Matrix into a cell dish in the laboratory and then add patient leukemia cells, they immediately are drawn to, attach themselves and become embedded. Upon evaluation, these attached cells were found to have stem-cell properties. We believe other cancers will behave the same way. This process of seeding cell dishes with Matrix disks is clearly demonstrated in our video that can be found on the Sanatela website www.sanatelamedical.com.
Once the leukemia cancer cells, or potentially other CSCs, are thriving in the Matrix, researchers can safely test a range of cancer treatments in vitro. In a typical case of leukemia cancer, 24 different combinations and dosages are currently tested in the lab at one time. The results are then analyzed to quickly and accurately identify the precise treatments that are most successful in killing the CSCs.
“As an oncologist who makes treatment decisions every day, the ability to test all the therapies and options and create a treatment plan in the lab – before I administer them to my patient – will be a major improvement,” says Dr. Aljitawi.
Advancing Precision Medicine
The days when cancer patients received one-size-fits-all regimens of chemotherapy and radiation may soon be a thing of the past. Instead, advanced technologies like the Sanatela Matrix will enable doctors to take a more nuanced view of what drugs and treatments will work on individual patients with different kinds of cancers.
The goal of the precision medicine, or personalized medicine, movement is to determine the exact drugs or treatments that have the best chance of working in every unique individual patient. To date, most of the advances in precision medicine cancer treatments have come from genetic testing.
Researchers using the Matrix will be able to create Individualized Cancer Screening and Treatment plans in vitro by taking a patient’s cancer stem cells and growing them in a 3D environment that substantially replicates the environment in the human body.
“Oncologists already have a number of readily available therapies to treat leukemia and most other cancers,” says Sanatela’s Co-Founder and CEO Michael Crowley, “but one of the biggest challenges has been to figure out which one will be the most effective. The Sanatela’ Matrix will allow oncologists and clinicians to demonstrate via lab tests which individualized therapies can potentially kill all of the cancer stem cells. Sanatela may be another important step forward toward the elusive goal of creating personalized cancer treatments in a lab. Not only that, the Matrix will help investigators identify new targets to develop effective new therapies.”
“While our researchers have made some incredible discoveries in the lab, the real breakthrough will happen once cancer patients are more effectively treated using the precisely targeted therapeutic products at cancer treatment centers and labs across the globe,” says Michael Crowley. “We are moving as fast as we can to get our new product into the hands of researchers and others who are interested in using the Matrix for cancer or stem cell research purposes, High-Throughput Drug Testing or whatever other purposes the researchers may use the product to create custom treatments designed in the lab.”
Future Uses
Providing innovative tools to help treat cancer is just one of many ways the Sanatela Matrix is making breakthrough medical advances. The Company also believes that the Matrix could potentially be applied by medical professionals as a highly effective wound dressing to open wounds or burns. Sanatela plans to perform clinical trials on the Matrix as a wound dressing in the near future.
Because the Matrix is porous and made exclusively of Wharton’s Jelly obtained from live birth human umbilical cord tissue, the Matrix should naturally degrade after being used as a wound dressing. Sanatela believes that its Matrix will not be expected to invoke an immune response and may facilitate skin regeneration as it degrades naturally.
Sanatela is seeking an investment of $5 million to shepherd its current and future products to the global biomedical marketplace.
“We believe the Matrix will have a decided advantage over other wound dressing products currently on the market,” says Sanatela’s Co-founder and Executive Vice President Erin Crowley Ellis. “It could potentially not only be used for wound dressings, but it could also be used in other situations that require the regeneration of tissue to replace a missing part of bone or skin."
"Sanatela has taken something that has largely been considered medical waste, umbilical cord tissue from live births, and bioengineered a material with intrinsic and highly valuable biological properties,” Erin Crowley Ellis says. “Sanatela is excited to be working on this breakthrough discovery and proud to help bring it to the world.“
About Sanatela Medical see www.sanatelamedical.com
Sanatela Co-Founder Bios
Founder Michael Crowley is a serial high-tech entrepreneur, international lawyer, tech venture capitalist, M&A professional and investment banker with strong personal track-record in the field of technology entrepreneurship and advanced technology commercialization. He is currently associated with both the U of R’s Simon Business School as a Mentor and RIT’s College of Engineering Technology, respecting a new technology entrepreneurship and commercialization joint certificate course being presented at ATVentureCenter. Mr. Crowley has previously been a Member of the Dean’s Advisory Council and a Research Professor of Innovation & Entrepreneurship at RIT’s Saunders College of Business and lecturer at the University of Maryland, College Park, Maryland China Initiative. Crowley is also an avid business and high-tech researcher, international transactions educator and seasoned professional speaker and lecturer who has trained over 500 international & Chinese business/science and technology delegations to the US. He is also the Co-Founder of ATVentureCenter™ (“ATVC”).
Erin Crowley Ellis, the co-founder Managing Director of ATVC and Sanatela, is a Toyota Management System trained mechanical engineer and businesswoman. Trained under expert Japanese Toyota Way guidance, Erin has a deep appreciation and understanding of quality systems, including advanced technology systems. Erin consults with Fortune 500 companies, along with hundreds of R&D firms and manufacturing companies in biomedical engineering, automotive manufacturing and materials testing. Erin is an honors graduate of RIT in Mechanical Engineering who brings deep quality management experience to Sanatela and other ATVC high tech commercialization businesses. For over ten years she has solved systemic production and quality problems while serving as a Lead Auditor: ISO 14001 Environmental/ISO 9001 Quality/IATF 16949 Automotive/ ISO 13485 Medical/ ISO 17025 Testing & Calibration. Erin is 1 of only 200 registered international automotive auditors- IATF 16949.
About ATVentureCenter, see www.atventurecenter.com
For further information, please contact:
Michael Crowley, Chairman and CEO, Sanatela AT Medical Solutions, Inc.
michaelcrowley@sanatelamedical.com
(585) 546-8311
Source: Sanatela AT Medical Solutions, Inc.