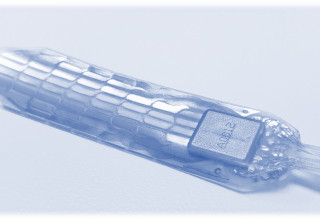
Micro-Leads Receives FDA IDE Approval for 60-Electrode Spinal Cord Stimulator System
The Company will launch its high-definition spinal cord stimulator study in human subjects.
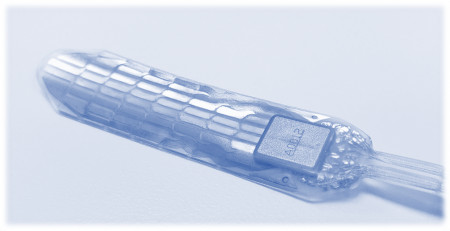
SOMERVILLE, Mass., May 21, 2022 (Newswire.com) - Micro-Leads Inc, a medical device technology company today announced FDA Investigational Device Exemption (IDE) approval for its high-definition spinal cord stimulation (HD-SCS) system.
Dr. Julie Pilitsis, MD, Ph.D., the neurosurgeon leading the clinical study, said "News of this approval is very welcome as it opens the door for improved non-opioid treatment options for those suffering with chronic pain. By reducing some of the current device limitations, such as the inability to target lateral spinal cord fibers, this device has the potential to double the number of therapy points and provide additional pain relief. Initial results showed a wide range of targeted fibers, which is very promising."
Over 1M people suffer from chronic focal pain: debilitating pain in a small body area that is disproportionate to the original injury. Severe focal pain can develop as a complication of knee or hernia surgery, common sprain injury, or progression of diabetes. Micro-Leads' HD-SCS provides a new ability to treat this type of focal pain by directly stimulating the lateral spinal cord pathway using a precision 60-electrode array system. Conventional spinal cord stimulators cannot reliably treat this kind of pain due to device targeting and procedural limitations.
Micro-Leads Founder and CEO Bryan McLaughlin, Ph.D., said, "FDA approval of our HD-SCS pulse-generator and lead system is an exciting development in our high-efficiency dosing platform for chronic pain management."
Earlier this year, Micro-Leads made a breakthrough 50% dose-reduction discovery while beta-testing their emerging needle-deployable directional stimulator device. "Most neuromodulation devices today only have a 30% dosing efficiency, resulting in a need to significantly overstimulate the nervous system. Therapeutic and device related complications from overstimulation include discomfort, pain, daily device recharging, and a larger implanted device battery."
"Our injectable technology reduces the stimulation dose required to treat by one-half," said Dr. McLaughlin. "These high-efficiency dosing improvements provide a unique and unprecedented opportunity to treat chronic focal pains, migraines, and diseases where conventional spinal cord and peripheral nerve stimulators have failed." Chronic focal pain alone represents a $1B patient population where conventional therapies suffer from unpredictable procedures, complications, and loss of efficacy.
About Micro-Leads Medical, Inc.
Micro-Leads is a medical technology Company developing a needle-deployable, high-efficiency neurostimulator platform for treating chronic focal pain and neurological conditions using directional dosing paradigms of the spinal cord and peripheral nerves. Micro-Leads has received funding from the National Institutes of Health under a Helping to End Addition Long-term (HEAL) award. Micro-Leads' active-lead devices are among the highest-density implant-grade medical-devices - potentially enabling new brain computer interfaces (BCIs) as well.
About Albany Med
Albany Med, northeastern New York's only academic health sciences center, is one of the largest private employers in the Capital Region. It incorporates the 766-bed Albany Medical Center Hospital, which offers the widest range of medical and surgical services in the region, and Albany Medical College, which trains the next generation of doctors, scientists and other healthcare professionals.
Contact
Bryan McLaughlin, Chief Executive Officer
Source: Micro-Leads Inc.