ivWatch Closes $14.8 Million Funding Round
HAMPTON, Va., June 7, 2017 (Newswire.com) - ivWatch LLC, a medical device company focused on improving the safety and effectiveness of intravenous (IV) therapy, today announced the closing of its latest funding round of $14.8 million from undisclosed angel investors, family funds and a major hospital system. The financing will support the company’s direct go-to-market initiatives and strategic partner activities.
“The support ivWatch has seen from our investors and partners has been incredible. They are committed to seeing our technology, research, science and team make a true impact on the problems related to peripheral IV failure,” says Gary P. Warren, president and CEO of ivWatch LLC. “The momentum we are finding in the market is exciting, as healthcare providers see the benefits of improved patient safety and reduced risks associated with IV therapy.”
"The support ivWatch has seen from our investors and partners has been incredible. They are committed to seeing our technology, research, science and team make a true impact on the problems related to peripheral IV failure. The momentum we are finding in the market is exciting, as healthcare providers see the benefits of improved patient safety and reduced risks associated with IV therapy."
Gary P. Warren, President and CEO, ivWatch LLC
IV therapy is the most common invasive hospital procedure. Yet failure rates due to infiltration are pegged at more than 20 percent. Every failure of an IV results in a drug delivery error and carries the potential for reduced drug efficacy and physical harm. Complications range from skin redness, burning, tissue necrosis and compartment syndrome to possible limb amputation.
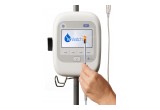
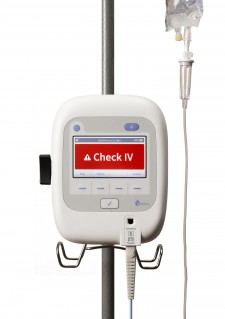
ivWatch technology is the first of its kind to offer a solution for continuously monitoring an IV site and assessing if drugs are actually being delivered to the vein without leakage. The ivWatch Model 400 is an FDA-cleared device that continuously monitors any patient’s peripheral IV for the early detection of these infiltration and extravasation events and helps clinicians manage the risk associated with IV therapy. The company also recently launched the ivWatch Original Equipment Manufacturer (OEM) Board to enable seamless integration of ivWatch’s groundbreaking IV infiltration detection technology with patient monitoring systems, infusion pumps and other devices. By integrating ivWatch technology into existing products, OEMs will be able to help healthcare customers deliver a higher level of patient care.
“ivWatch represents the entrepreneurial progress of our region,” says John Lawson, president and CEO of W.M. Jordan Company and chairman of the board at ivWatch LLC. “The scientific, financial and business acumen of those involved with the company, from investors, board members and advisors to the employees, is remarkable. Every day, the team moves one step closer to helping resolve the infiltration issue within our healthcare system.”
For more information about ivWatch and its revolutionary medical technologies, go to www.ivWatch.com/products.
About ivWatch
ivWatch LLC is a medical device manufacturer focused on improving patient safety and the effectiveness of intravenous therapy. With the Model 400, which is similar to continuous monitors available for a patient’s heart, blood oxygen level and pulse, ivWatch provides a first-of-its-kind, FDA-cleared, noninvasive medical device that continuously monitors peripheral IVs for infiltration and extravasation events. The ivWatch Model 400 aims to help eliminate patient harm associated with adverse events and to reduce drug delivery errors. The ivWatch OEM Board integrates ivWatch technology into existing products, enabling OEMs to help healthcare customers deliver a higher level of patient care and to reduce risks related to IV therapy. Follow us on Twitter@ivWatch or Facebook @ivWatchLLC. www.ivWatch.com
MEDIA CONTACT: Leah Moore, leah.moore@ivwatch.com, Office (855) 489-2824 x7031, Mobile (757) 839-5653
The ivWatch OEM Board is not an FDA-cleared device. It is the responsibility of the Original Equipment Manufacturer integrating ivWatch technology to secure the proper regulatory clearance.
Source: ivWatch LLC