Xavant Technology Announces First Dual-Sensor Neuromuscular Patient Monitor
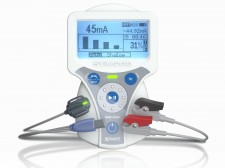
PRETORIA, South Africa, October 15, 2019 (Newswire.com) - Xavant Technology, a pioneer in neuromuscular monitoring, stimulation, and innovative neuromodulation modalities, announced an addition to the company’s newest generation of Stimpod neuromuscular transmission monitor - the capability of utilizing either of the two most industry-prominent types of monitoring sensors: AMG and EMG. The new Stimpod system and EMG sensor accessory will be exhibited at the American Society of Anesthesia (ASA) Annual Meeting Oct. 19-21 in Orlando, Florida, alongside the company’s entire Stimpod portfolio for anesthesia.
"We are excited to announce the EMG modality to our Stimpod line of monitors," stated Corlius Birkill, CEO of Xavant Technology. "By offering, for the first time, anesthesiologists and clinicians a choice in using either AMG or EMG, we can give them unparalleled clinical and budgetary benefits. We believe quantitative or objective monitoring of patients who are undergoing neuromuscular block for surgery should be the standard of care. Our goal is to provide physicians with the most optimal and efficient tools to achieve that standard.”
The latest update to the AMG-based Stimpod NMS450X monitor series will enable the use, for the first time ever, of a dual-sensor objective neuromuscular transmission monitor that enables anesthesiologists the choice of using either acceleromyography (AMG) with a reusable sensor or electromyography (EMG) with a disposable sensor to manage patients undergoing neuromuscular block during surgery or while being cared for in the intensive care unit.
By adding an EMG sensor accessory to the Stimpod, clinician opportunities in monitoring will be maximized. Being able to choose either AMG or EMG at the site of service, hospitals can perform cost-effective entire-surgery monitoring with the platform that is optimal for that specific case. While AMG is a proven, accurate and cost-effective technology, the EMG sensor will simplify how clinicians monitor patients in more restrictive surgical cases, such as robotic surgery where restricting the hands is common. The EMG accessory is pending FDA clearance.
"The Stimpod NMT monitor is a simple and economical way for hospitals to drive patient safety, operating room, PACU, and ICU efficiency, and manage their very expensive paralytic and recovery drug budgets,” stated Xavant Chairman Roche van Rensburg. “We believe the data is fairly conclusive that hospitals can enhance safety outcomes related to residual neuromuscular block by utilizing objective NMT monitoring. But also important is the power to more effectively manage the time and cost-of-care efficacy for the hospital – we believe the Stimpod system can make a tremendous positive difference on both fronts.”
About Xavant Technology:
Xavant Technology (Pty) Ltd is a leading manufacturer and developer of neuromuscular monitors, stimulators, and treatment devices for regional anesthesia, general anesthesia, and pain management applications. Learn more about Xavant Technology at www.xavant.com.
Media Contact:
Lourie Höll
Email: media@xavant.com
Source: Xavant Technology
