Victory Nutrition International Inc. (VNI) Announces Results of a Placebo-Controlled 1-Way Crossover Clinical Trial on a Finished Product
LEDERACH, Pa., June 4, 2019 (Newswire.com) - Victory Nutrition International, Inc (“VNI”) announces Prodovite VMP35 MNC, a novel iron-free supplement, enhances cytoprotection against anemia in human subjects: a novel hypothesis. The clinical study paper is published in Food & Nutrition Research, A Peer-Reviewed Journal in PubMed. https://www.ncbi.nlm.nih.gov/pubmed/31105509
VNI Scientists developed a novel concept that iron-deficiency in Red Blood Cells is not actually caused by an iron deficiency, but by a deficiency in alkaline buffers. A pilot clinical study was conducted in 38 human subjects (10 men and 28 women, ages 22 - 82) to evaluate the rate of absorption and effects on blood of VMP35 multi-nutrient complex (MNC), a non-iron containing liquid nutraceutical supplement. After taking a baseline blood sample (at ‘0’ minutes), subjects consumed either a placebo or the patent-pending iron-free SK713 Prodosomed VMP35 (30 mL). Their blood was then evaluated at 5 and 30 minutes post intake. This pilot clinical study confirmed and validated that the iron-free VMP35 rapidly restored iron-dependent hemoglobin to RBCs.
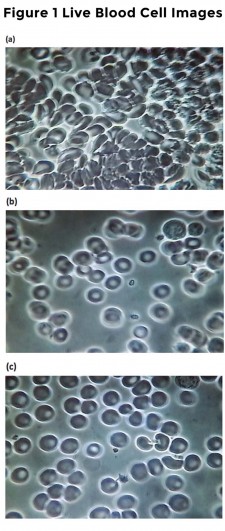
Figure 1. Baseline and 5 and 30 min after VMP35 supplement. Fig. 1a. Live blood cell imaging of subject #45 3STILL1BM45 (baseline before VMP35 supplementation). Fig. 1b. Live blood cell imaging of subject #45 3STILL3AM45 (5 minutes after VMP35 supplementation). Fig. 1c. Live blood cell imaging of subject #45 3STILL1THIRTY45 (30 minutes after VMP35 supplementation).
The non-iron containing VMP35 can induce improvements in blood properties and potential benefits for subjects even with compromised digestive systems. No adverse events were reported. Further research studies are in progress to explore the mechanistic insight.
- Red Blood Cell (RBC) and blood rheology (flow) improvements were observed demonstrating that VMP35 encapsulated in multilamellar SK713 SLP Prodosome technology was delivered and absorbed by sublingual trans-mucosa within 5 minutes.
- Adhering to meticulous published standards, Live Blood Cell Imaging (LBCI) of a Peripheral Blood Smear (PBS) offers a unique ability to observe the rapid onset of changes in properties of the blood in response to interventions.
- Changes were observed within 5 minutes of VMP35 administration, which was sustained for at least 30 minutes. Group 2 at 5 minutes and Group 3, at 5 and 30 minutes support the validity of the observations. No effect was observed in the control group. Prompt, sustained and progressive findings were observed in the Treatment groups.
- Non-genetic and non-hemorrhagic anemias conventionally termed ‘Iron Deficiency Anemia’, are characterized by deficiencies in alkaline buffers and the expenditure of histidine from hemoglobin to act as an alkaline buffer. This releases iron, which is repartitioned to other tissues like the liver, bone marrow, etc. The authors coined a new more accurate and descriptive term for this process called “Chronic Anemia Syndrome” (CAS), since the iron is still in body tissues.
- Overall, the SK713 SLP delivery technology of the iron-free VMP35 MNC was shown to exert a rapid positive response on the form, structure, function, and flow properties of the blood, rapidly restoring hemoglobin to RBCs, which strongly suggests a paradigm shift in the understanding, diagnosis, and treatment of Iron Deficiency Anemia.
Food & Nutrition Research (FNR)
As one of the first Open Access journals in its field, Food & Nutrition Research (FNR) offers an important forum for researchers to exchange the latest results from research on human nutrition broadly and food-related nutrition in particular. FNR is widely indexed by relevant services and databases, including PubMed Central/PubMed, Scopus and Science Citation Index. This current research paper can be downloaded directly from Pubmed. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6510707
For more information about Food & Nutrition Research,
please visit: https://foodandnutritionresearch.net
About PRODOVITE®
PRODOVITE is VNI’s patent-pending Multivitamin Mineral and Phytonutrient Complex. PRODOVITE’s Prodosome® Encapsulation Technology promotes rapid, synchronized and sustainable absorption of its nutritional ingredients. The results of this study confirm that PRODOVITE can induce improvements in blood properties, unlike other multi-nutrient products.
For more information on PRODOVITE® please visit https://vni.life/retail/corporate/product/17908
About Victory Nutrition International, Inc. (VNI)
VNI was launched in January 2014 and its founders are biochemists, formulators and published researchers. VNI Produces high-quality well-researched products with unique, exclusive and patent-pending formulas. Their first-to-market products are made with premium quality research-driven, safety affirmed ingredients encapsulated in an advanced absorption technology. VNI products are validated by published clinical studies.
For more information please visit www.vni.life
Contact Information
Bill Downs
Victory Nutrition International, Inc.
Founder and CEO
(215) 872-3334
email: billd@vni.life
Jeff Hooks
Victory Nutrition International, Inc.
President and COO
(919) 868-6988
email: jeff@vni.life
Press Contact:
Suzanne Brady
(866) 881-1624
email: Suzanne@vni.life
Source: Victory Nutrition International
