Prism Medical & Design Earns FDA Clearance for Its ProteXsure Safety Capsule System- Looks to Add International Distributors
Coral Springs, Florida USA, July 6, 2016 (Newswire.com) - The FDA has granted Prism Medical & Design clearance for its ProteXsure Safety Capsule System, the Coral Springs, Fl. based company announced today. The System, which earned CE Marking in February, reduces dangerous and sometimes fatal needle sticks for at risk health care workers, caregivers, consumers and downstream waste management workers.
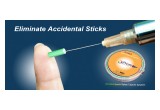
The “system” is patented, disposable and contains 100 protective needle capsules that are used to cover a needle tip after injection to prevent Needle Stick Injuries (NSI’s) from happening. Globally, there are 3.5 million reported cases of NSI’s annually, with 70% of NSI’s occurring while disposing of the needle. In the U.S. alone, there’s roughly 1 million NSI’s reported annually with an associated cost of $3.5 billion to treat. NSI’s are an epidemic and lead to life threatening diseases such as: Hepatitis B, C, and HIV.
Prism Medical & Design earns FDA Clearance for its ProteXsure Safety Capsule System, looks to add international distributors
Les Capella, Principal
The ProteXsure Safety Capsule System is automated, requires only one hand to operate and automatically advances to the next capsule after a needle is inserted into the device and covered. The ProteXsure Safety Capsule System is the first of its kind and one of many patented sharps safety devices that Prism will introduce to the global markets in 2016. It’s Prism’s mission to protect and redefine NSI’s by developing easy to use, safe devices through advanced engineering designs.
“The FDA Clearance, awarded for the ProteXsure Safety Capsule System, is another indication of the Prism platform”, Les Capella & Chip Starnes said in a prepared statement. “We look forward to continuing our global launch and establishing our global footprint with exclusive partners in the protection of health care workers & consumers using our family of sharps safety devices”.
http://www.PrismMedicalDesign.com
Source: Prism Medical & Design