GeneTex Develops Recombinant Influenza Nucleoprotein Antibodies as Potential Diagnostics
IRVINE, Calif., August 2, 2021 (Newswire.com) - GeneTex, a leading international antibody manufacturer, has established a reputation for providing highly validated research antibodies to virologists with extensive antibody portfolios for SARS-CoV-2, Zika virus, Dengue virus, and other viral pathogens. The Company has now expanded its existing influenza virus catalog with the release of a series of recombinant rabbit monoclonal antibodies that broadly detect the nucleoproteins (NPs) of influenza A and influenza B strains. Though conceived as tools for research, these antibodies also show promise for diagnostics development.
Both influenza A and influenza B viruses are responsible for potentially lethal human disease and epidemics, with influenza A associated with pandemics. Of the many influenza A strains identified by their respective neuraminidase and hemagglutinin surface antigens, only the H1N1 and H3N2 viruses are linked to sustained transmission between humans. In the quest to create new antiviral therapies and universal vaccines, interest has focused on the viral NP given its multiple functions in viral RNA structure, nuclear trafficking, and replication as well as its high degree of sequence conservation. Thus, antibody reagents targeting this protein have significant value for influenza researchers, vaccine biologists, and possibly for diagnostic kit manufacturers.
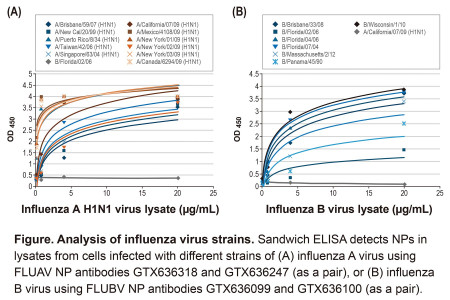
The new influenza A and B antibodies were created in GeneTex's recombinant antibody facility and join the Company's existing catalog of rabbit polyclonal and mouse monoclonal reagents against different influenza proteins. There are three recombinant antibodies (GTX636199 [HL1078], GTX636247 [HL1089], GTX636318 [HL1103]) for influenza A NP and three (GTX636099 [HL1068], GTX636100 [HL1069], GTX636194 [HL1073]) for influenza B NP. GeneTex's in-house testing found that all six antibodies perform well in indirect ELISA. In addition, four combinations of the influenza A antibodies (as capture/detection pairs) recognize the NPs of at least sixteen H1N1 and H3N2 strains with picomolar sensitivity in sandwich ELISA (sELISA). All three influenza A antibodies were able to detect the NPs of all or almost all of the same strains by western blot with negligible background. For the influenza B antibodies, two antibody pairs were found to perform in sELISA for detection of the NPs from at least nine influenza B strains, and all three antibodies were validated for western blot.
These recombinant influenza NP antibodies mark another milestone in GeneTex's efforts to support research combating pathogenic viruses, both endemic and emerging.
GeneTex products are for research use only. Not for diagnostic or therapeutic procedures.
Allen Lee
Phone: 949.553.1900
Email: allensl@genetex.com
Source: GeneTex, Inc.
