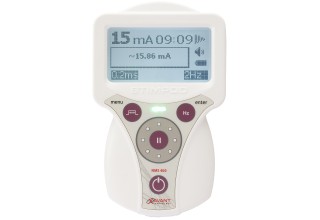
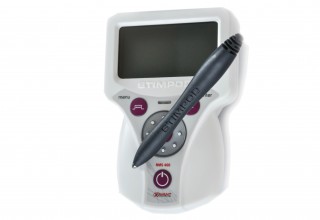
FDA Clears Xavant Technology's Stimpod NMS460 Non-Invasive Neuromodulation Device for Treatment of Chronic Intractable Pain
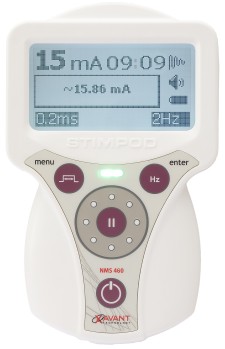
Pretoria, South Africa, August 1, 2017 (Newswire.com) - Xavant Technology (Pty) Ltd today announced that the U.S. Food and Drug Administration (FDA) cleared their Stimpod NMS460. With the U.S. patent awarded on proprietary hybrid pulsed radio frequency (PRF) waveform, this non-invasive neuromodulation device is focused on the symptomatic relief and management of chronic intractable pain, as well as an adjunctive treatment in the management of post-surgical pain, post-traumatic acute pain problems, and an adjunct for pain control due to rehabilitation.
The need for this device is profound. More than 100 million Americans report that they have a problem with pain.[1] Chronic pain is characterized by neuroplastic changes that cause sensitization of the nervous system. These changes result in anatomical and physiological changes that affect neurological function, which causes long-term potentiation and gene expression changes that then allow the pain to continue with or without further peripheral input, and a lower pain threshold; this dysfunction then also accounts for the epiphenomena associated with the disease, including emotional, memory, and motor changes, which then becomes the illness of chronic pain.[2]
We are thrilled at the news that our revolutionary device can now be used in the U.S. This ground-breaking technology has the ability to help tens if not hundreds of millions of people just in the U.S. as a valuable treatment asset for neurologists, chiropractors, acupuncturists, physical therapists, physiatrists, and medical pain practitioners.
Corlius Birkill, CEO of Xavant Technology (Pty) Ltd
Treatment of chronic pain in the United States is estimated to cost $600 billion annually.[3] The current standard of care for chronic pain includes drug cocktails such as Corticosteroids, Opiate pain relievers, injections, and beyond combined with treatments like physical therapy and counseling. Treatments can come with various short-term and long-term side effects.
The Stimpod NMS460, however, will greatly impact the industry as a non-invasive, non-drug solution with zero side effects and a fast onset of effect at a fraction of the cost of comparable treatments. The device applies its patented PRF waveform to the affected area transcutaneously. This waveform creates electromagnetic effects similar to invasive pulsed radio frequency treatments, and several case studies have shown instant and dramatic relief of chronic intractable pain. The Stimpod NMS460 also incorporates the nerve-locating technology which features a nerve mapping probe that enables practitioners to locate nerves and evaluate the treatment progress of damaged nerves. Bell Medical has been selected as the master distributor for both the U.S. and Canada.
"We are thrilled at the news that our revolutionary device can now be used in the U.S.," said Corlius Birkill, CEO of Xavant Technology (Pty) Ltd. "This groundbreaking technology has the ability to help tens if not hundreds of millions of people just in the U.S. as a valuable treatment asset for neurologists, chiropractors, acupuncturists, physical therapists, physiatrists, and medical pain practitioners."
About Xavant Technology (Pty) Ltd
Xavant Technology (Pty) Ltd is a leading supplier of nerve stimulators for regional and general anesthesia applications. The current Stimpod range has three models - the NMS410, NMS450 and NMS460. The Stimpod NMS460 is a neuromodulation device used for symptomatic relief of chronic intractable pain, implementing a unique patented Pulsed Radio Frequency waveform for the treatment of neuropathies. The Stimpod NMS410 model is a specialized nerve locating device, with its unique nerve mapping and locating cable, used primarily during Regional Anesthesia procedures. The Stimpod NMS450 adds the option to monitor neuromuscular blocking agents with its advanced three-dimensional accelerometer. Learn More About STIMPODNMS460 at http://www.stimpodnms460.com.
Media Contact:
Lourie Höll
Phone: +27 12 743 5959
Email: media@xavant.com
[1] Gaskin, Darrell J., and Patrick Richard. "The Economic Costs of Pain in the United States." The Journal of Pain: Official Journal of the American Pain Society (2012): 715-24. Web. 21 June 2017.
[2] American Academy Of Pain Medicine. Neuropathic Pain Recognized as a Disease. N.p.: American Medical Association, 27 Sept. 2016. PDF. https://assets.ama-assn.org/sub/meeting/documents/i16-resolution-912.pdf
[3] Gaskin, Darrell J., and Patrick Richard. "The Economic Costs of Pain in the United States." The Journal of Pain: Official Journal of the American Pain Society (2012): 715-24. Web. 21 June 2017.
Source: Xavant Technology (Pty) Ltd