Belhaven Biopharma to Present Promising Phase 1 Data on Needle-Free Epinephrine Powder at ACAAI Annual Meeting
RALEIGH, N.C., October 24, 2024 (Newswire.com) - Belhaven Biopharma, a leader in nasal drug delivery technologies and emergency allergy care solutions, is set to present new clinical data from its Phase 1 study on Nasdepi®, an intranasal epinephrine powder, at the 2024 American College of Allergy, Asthma & Immunology (ACAAI) Annual Scientific Meeting. The presentation, scheduled for Friday, October 25, at 2:15 p.m. (ET), will take place in Exhibit Hall A, Monitor 28, at the Boston Convention & Exhibition Center.
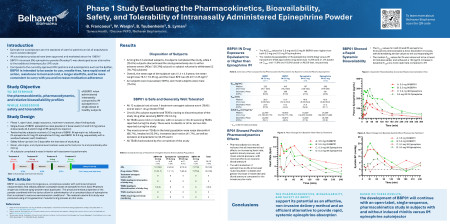
Titled “Phase 1 Study Evaluating the Pharmacokinetics, Bioavailability, Safety, and Tolerability of Intranasally Administered Epinephrine Powder,” the presentation will reveal findings that demonstrate Nasdepi®’s potential as a safe, non-invasive alternative to traditional intramuscular epinephrine administration. These results are especially relevant for severe allergy emergency settings, where rapid and effective intervention is critical.
Key findings:
- Sustained Drug Exposure: Nasdepi® (3.5 mg and 5.5 mg doses) provided greater overall drug exposure than both 0.3 mg and 0.5 mg intramuscular (IM) epinephrine.
- Rapid Absorption: Nasdepi® reached effective and peak concentrations in the bloodstream 48% and 22%, respectively, faster than the FDA-approved IM epinephrine dose, positioning it as a highly responsive option for emergency treatment.
- Alternative to Intramuscular Injections: The PK, bioavailability, and safety of Nasdepi in this Phase 1 Study support its potential as an efficient alternative to rapidly deliver epinephrine systemically to treat severe allergic reactions including anaphylaxis.
“We are excited to share our clinical data with the ACAAI community,” said Scott Lyman, CEO of Belhaven Biopharma. “These results affirm our ability to develop novel life-saving treatment options. Nasdepi® represents a breakthrough in epinephrine delivery by offering an easy-to-use, temperature-resistant, and needle-free solution for emergency allergy care.”
The study compared Nasdepi® to conventional intramuscular (IM) epinephrine in healthy subjects, highlighting comparatively rapid increases in plasma concentration to meaningful plasma levels such as the time to achieve a 100 pg/mL increase above baseline (T100) and equivalent or higher Cmax versus IM injection—key factors in time-sensitive allergic reactions. Additionally, Nasdepi® exposures were accompanied by desirable moderate increases in heart rate and systolic blood pressure confirming systemic distribution. Treatments were generally safe and well tolerated. No serious adverse events were reported and treatment-related events were resolved by the end of the study. All results underscore Nasdepi®’s potential as a viable alternative to IM epinephrine.
New research on Nasdepi®’s dry powder formulation, showing reliable deposition under real-life conditions like nasal congestion and its stability under extreme heat, will be presented by Belhaven Biopharma at the Drug Delivery to the Lungs (DDL) Conference on December 12 in Edinburgh, Scotland. Chief Operations Officer Brian Taubenheim will discuss these findings, underscoring Nasdepi®’s potential as a dependable, needle-free option for emergency allergy care. More details are available at DDL Conference.
For more information, please visit belhavenbio.com.
About Belhaven Biopharma
Belhaven Biopharma is a clinical-stage pharmaceutical research company specializing in developing life-saving medications delivered quickly, effectively, and painlessly with a simple, dry powder, single-use nasal device. They are at the forefront of developing nasal dry powder epinephrine, which is revolutionizing emergency-use epinephrine delivery and expanding global access. Nasdepi®, Belhaven's lead program, is the first dry powder nasal device for treating life-threatening allergic reactions.
Source: Belhaven Biopharma
